

Ready to automate your future? Get a quote from Norck Robotics now!

Norck Robotics specializes in providing unique robotic automation and engineering solutions designed to meet the specific operational needs of each client. Our expertise covers a wide range of industries and applications.

Norck Robotics delivers turnkey robotic automation and engineering solutions tailored to your specific needs across various industries.

Whether you need a single robotic cell prototype or full-scale factory automation, Norck Robotics engineers are ready to collaborate with you to bring your concept to life.
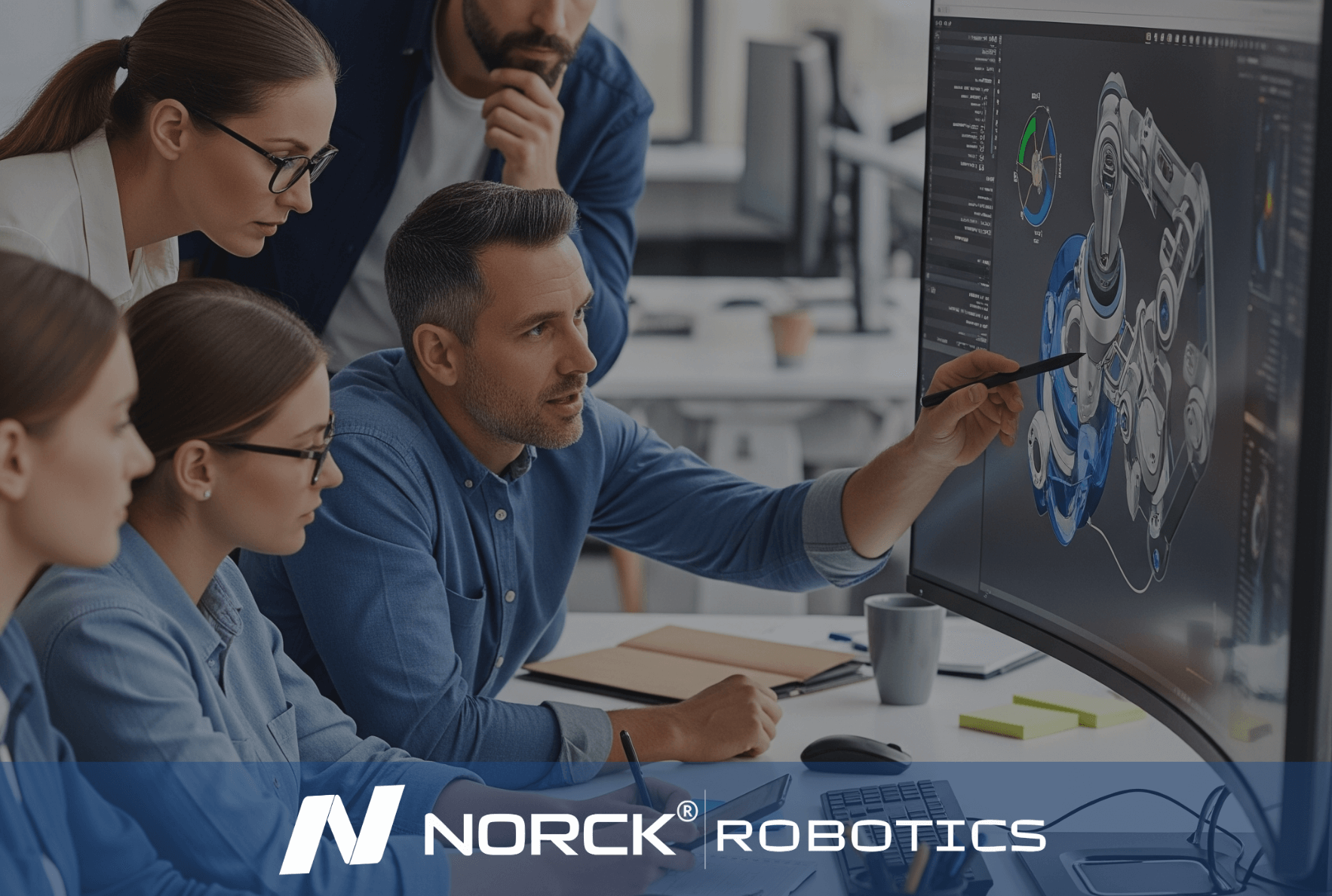
Norck Robotics engineers analyze your existing processes to provide feedback that enhances efficiency, cost-effectiveness, and productivity for robotic integration.

Biocompatible materials interact safely with the body’s tissues and fluids without provoking cytotoxicity, inflammation, thrombosis or immune rejection. They must pass standardized tests (ISO 10993 series) for cell viability, sensitization, irritation and hemocompatibility. In practice, this means no leaching of toxic ions, minimal protein adsorption and a surface finish that resists bacterial adhesion—essential for implants, catheters and any device in prolonged contact with patients.
High-temperature tolerance (up to 260 °C), chemical resistance to bodily fluids and sterilants; ideal for spinal implants and instrument components.
Ultra-low corrosion rates, excellent bone integration for implants, and compatibility with MRI fields.
High strength, good fatigue resistance; used in surgical tools and orthopedic hardware.
Outstanding wear resistance for joint replacements; retain surface hardness after sterilization.
Economical disposables, autoclavable or EtO/gamma tolerant; common in syringes and tubing.
Soft, elastic seals and catheters; withstands repeated steam, EtO or radiation cycles without embrittlement.
Extremely hard, bioinert for dental crowns and hip bearings; minimal wear debris.

In addition to its own expert engineering team, Norck Robotics provides access to a network of hundreds of top-tier system integrators, robot manufacturers, and component suppliers across the United States, Germany, and Europe.

Working with Norck Robotics reduces dependency on manual labor, increases production consistency, and secures your operations against unforeseen disruptions, quality issues, and fluctuations. This enhances your company's supply chain resilience.
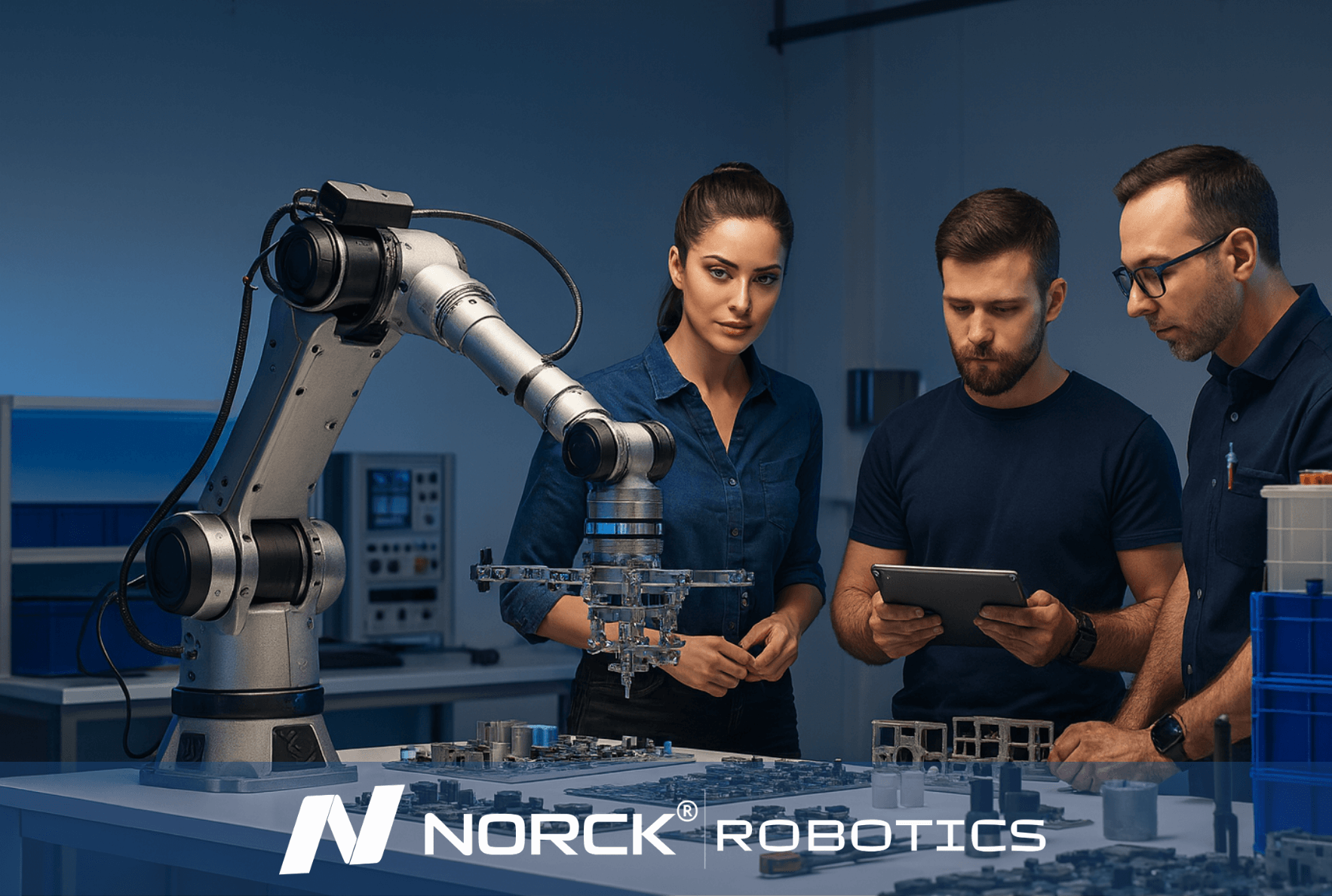
Norck Robotics advances digital automation by developing custom-designed robot grippers, advanced vision systems, and innovative simulation software. With an AI-driven, data-centric approach, it enables smarter system design, optimal performance, and predictive maintenance solutions.
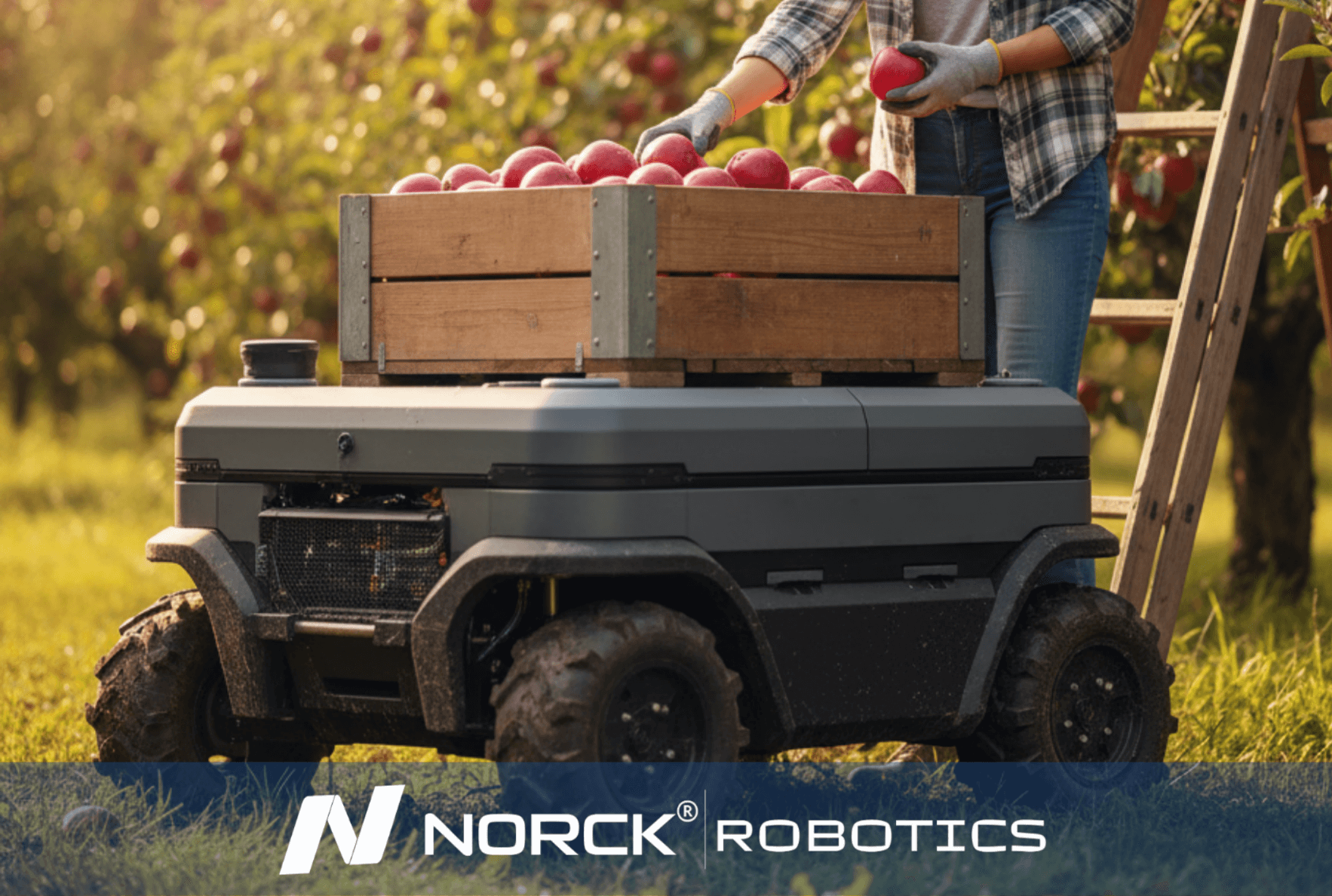
Norck Robotics encourages its partners to be carbon-neutral by reducing energy consumption and material waste through the efficiency of robotic automation, and prioritizes environmentally conscious suppliers.
Choosing the correct biocompatible, sterilizable material avoids corrosion fatigue and prevents particulate shedding that can trigger inflammation or block microfluidic channels. It ensures seals and moving parts retain tolerances, so kinematic performance remains within spec over years of use. Proper materials also simplify validation—fewer change notices, streamlined regulatory submissions—and lower total cost of ownership by reducing maintenance, leaks and device rework, all while safeguarding patient outcomes.